The Incredible Shrinking Cup

If you are a teacher, shrinking polystyrene cups is a fun way to demonstrate the impact of pressure on volume (i.e., Boyle’s Law) with your class. Students will see firsthand the effects of increased water depth on pressure and volume. The results can be quite striking! The Incredible Shrinking Cup lesson guides each student in developing a hypothesis about what will happen to their cup; calculating the cup’s mass, volume, and density; and learning how water pressure rapidly changes with depth.
Though the Great Lakes aren’t as deep as the ocean, you can shrink your cups in the deepest sections of the lakes (typically Lake Superior) through a unique partnership with Illinois-Indiana Sea Grant and EPA’s research vessel, the Lake Guardian.
This lesson is for high school students and has also been adapted for middle school and elementary school students.
See Q&A information below to learn how to participate in this program.

Scientists on board the R/V Lake Guardian deploy the cups into deep waters of the Great Lakes.
Additional Resources
- Check out The Incredible Shrinking Cup Lesson Materials for high school, middle school, and elementary school students.
- The shrinking cup video above might be of interest as an auxiliary resource, as well as this video explaining Boyle’s Law.
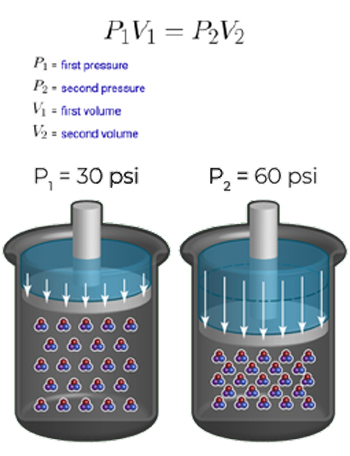
Boyle’s Law states that the pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
 Questions & Answers
Questions & Answers
What should I do if I am interested in this program?
- Email Janice Milanovich to tell her you’re interested. She will let you know what the available dates are for participating, which are dictated by the ship’s schedule.
- We’ll only be able to handle up to 25 cups per educator. This may require you to break up your students into groups and each group decorates its own group cup.
What should I do to prepare and then ship the cups?
- Don’t use waxy cups as they don’t shrink well.
- Have students decorate the cups with permanent markers so the colors don’t wash off.
- Have students write YOUR (teacher’s) initials on the bottom of their cups so that we can keep them all organized. Otherwise, we’ll have no idea if it belongs to you.
- Fill out the shipping label for the return shipment and include inside your box with the cups.
- Fill out the online form when you’re ready to mail the cups. This information helps us keep track of your cups and is used in our reporting.
- Lastly, send your 25 (or fewer) cups to:
Kristin TePas
USEPA Great Lakes National Program Office
77 W. Jackson Blvd. (G-9J)
Chicago, IL 60604-3511
How and when are the cups returned to me?
- Once the cups arrive at the EPA office, they will be transported to the ship. The cups will then be deployed at a deep station on one of the Great Lakes (usually Lake Superior). When the science survey ends, we will retrieve the cups from the ship and then send them back to you. Typically, it is about a month from when we receive the cups to when you get them back.
- When the cups are returned, we also will provide the actual depth to which the cups were deployed, so that you and your students can finish the post-deployment calculations in the lesson.
How can I share on social media?
Please tweet about your cups! We love sharing teachers’ posts about their Incredible Shrinking Cup activities. If you tweet about this, please tag @ILINSeaGrant and @EPAGreatLakes, and use the hashtag #IncredibleShrinkingCups in your post. If you aren’t on Twitter, please tag @ILINSeaGrant on Facebook or Instagram, or email a few photos to iisg@purdue.edu so that we can share for you.
We were so excited to take part in the Shrinking Cup lab with the @EPAGreatLakes @LakeGuardian! We were surprised to find that our cups were still in tact, just slightly smaller. Thank you @ILINSeaGrant and @EPA for connecting real world science for our @NOCSEagles! @CGLLiteracy pic.twitter.com/BQWuzlMITZ
— Shari Insley (@SInsley4) May 4, 2021
Contact Info
Great Lakes Literacy and Workforce Development Specialist
Related News
- Four Illinois and Indiana educators will set sail on Lake Michigan aboard EPA’s research ship
- New crayfish curriculum engages students in Great Lakes and local invasive species issues
- Spring brings a program review as well as education and outreach opportunities
- New aquaponics curriculum brings STEM and sustainable agriculture to the classroom
- Educators! Set sail on the 2025 Shipboard Science Workshop on Lake Michigan